Strip Film Manufacture
for Controlled Actives Delivery
Test bed lead: Rajesh Davé, NJIT
TESTBED 2
Research /

Continuous manufacture process of polymer strip films loaded with poorly water soluble API particles engineered for bioavailability enhancement
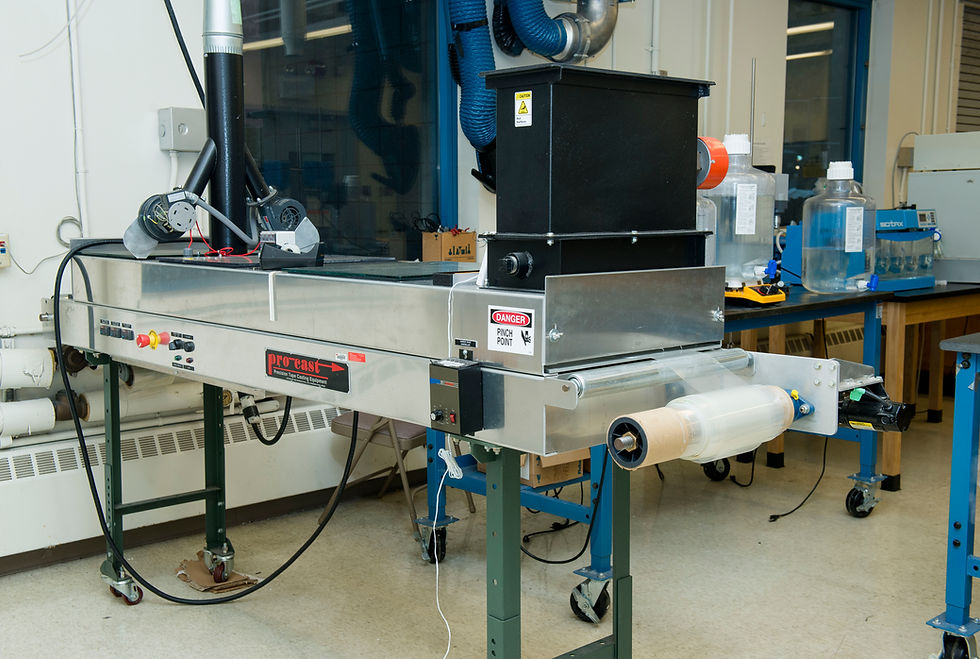
HED Film Casting line
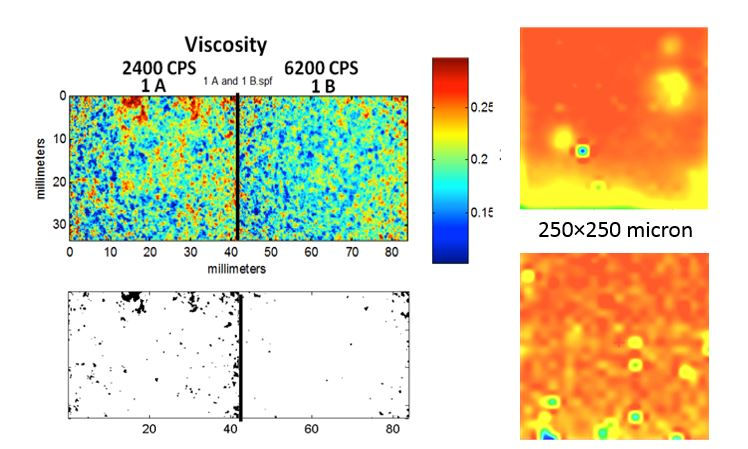
NIR chemical imaging for assessment of content uniformity of hydroxypropyl methylcellulose films loaded with griseofulvin nanoparticles.

Continuous manufacture process of polymer strip films loaded with poorly water soluble API particles engineered for bioavailability enhancement
Summary
Polymer films have emerged as a promising platform for the delivery of pharmaceutical products in recent years. The films are formed via solvent-casting, or in some cases hot-melt extrusion (HME). They are roughly the size of a postage stamp and can be placed on the tongue for immediate release or under the tongue (sublingual) or on the inside of the cheek (buccal) for sustained release. The majority of pharmaceutical films on the market are orodispersible, easily swallowed without water, and have improved compliance among pediatric, geriatric, and dysphagic patients.
These films offer several distinct advantages over traditional ODDs: (1) larger available surface area for rapid wetting, disintegration/dissolution; (2) more flexibility and less fragility than most orally disintegrating tablets; (3) precision in the administered dose as compared to drops or syrup formulations; (4) smaller size and convenience for anytime usage; and (5) less prone to chewing as compared to tablets or mini-pills. FDA approved dosages have included drugs such as ondansetron for nausea/vomiting, Suboxone® sublingual film containing buprenorphine+naloxone for the treatment of adult opioid dependence, and rizatriptan, an oral quick release film for migraines. One thing that all of these have in common is their use of water soluble drugs and relatively low dosage (< 10 mg), which are easiest to formulate with for fast dissolution and relatively homogeneous drug distribution.
Dozens of active pharmaceutical ingredients (APIs) are being developed for incorporation into strip films, and for many, ANDAs (abbreviated new drug applications) have been filed or will be filed soon. Some of the new developments include poorly water soluble APIs and/or much higher dosages. Unfortunately, most of the literature to date including patents, with the exception of the work done by our group within the C-SOPS, does not address the issues concerning poorly water soluble drugs and higher drug loadings. In addition, published papers and issued patents concerning the platforms (e.g., Pharmfilm®, RapidFilm®, and VersaFilm™) target the formulations or compositions of polymers and do not discuss manufacturing and quality related issues. The candidate drugs come from new uses for existing drugs (repurposing) or new formulations for existing drugs (life-cycle management), rather than exploitation of the true potential of the film format. Thus, they lack development of scientific understanding of the material properties, processes, or proper standards for product testing. In fact, two recent major review papers present little discussion on process understanding, indicating that engineering and regulatory aspects have been largely ignored.
Goals
The main goal of this test bed is to create an integrated manufacturing platform to manufacture film based products with precisely controlled release profiles.
It has an important role to play in C-SOPS as a natural platform for continuous manufacturing and quality by design of pharmaceutical products having predictable performance based on particle and other material properties, and has a high potential to become a real-time release platform.
The strip film format has several advantages including rapid disintegration and dissolution in the oral cavity and increased bio-availability, and is well suited for uniform dose manufacturing of high potency and/or poorly water soluble drugs. It may be further developed for applications such as pediatric and geriatric medicine, extended release, abuse-proof formulations, buccal delivery, as well as neo-natal therapeutic applications.
Key Accomplishments
Some of the key accomplishments of this test bed are:
-
In addition to nano-milled, crystallized, or dry milled drug particles, films can also be made using melt emulsions of poorly water soluble, low melting point drugs. In all cases, recovery of original drug particle size is achieved for multiple drugs, leading to immediate release for thin films.
-
Films with wide range of drug loadings (5-60 wt% API) possible.
-
Pullulan-based strip films, as alternative to HPMC-based strip films, also led to fast re-dispersibility of drug nano-particles and immediate drug dissolution.
-
Full nano-particle re-dispersibility and fast drug release, even for surfactant-free films.
-
Thick films (up to 2 mm) were produced via either casting a single thick film or multiple thinner films stacked and glued. Both achieved similar extended release dissolution profiles as thickness increased (zero order release for films > 1 mm).
-
Thick HPMC with xanthan gum films loaded with dry coated, milled fenofibrate particles were prepared for sustained release applications, where a linear relationship between film thickness and release time (varying from 10 to 1000 min) was observed.
-
A novel film fabrication approach was developed where multiple layers were cast in sequence to prepare layer-by-layer “sandwich” (LBLS) films. Controlled release films were produced by using water insoluble polymer for two outer layers.
-
A general framework, validated via off-line and in-line experiments, was developed to use Raman spectroscopy for monitoring the composition of film samples containing fenofibrate or naproxen.
-
Use of NIR and Raman spectroscopy as PAT tools was demonstrated as part of a quality-by-design toolbox for the manufacturing (casting and drying) of strip films for on-line non-destructive testing with a potential for real-time product release.
-
Real-time, in-situ surface dissolution UV imaging methodology is developed for discernment of the impact of various formulations and drug particle properties.
Impact
This testbed benefits industry by providing, for the first time, a systematic approach to developing films containing stable, well-dispersed nano and micro drug particles in edible or bio-compatible polymer films along with the guidelines for the design and characterization of these films. This is a novel platform and will have a major impact on the portfolio of dosage forms considered. Industry will also learn about the interactions between polymer, drug and surfactant in the film and how these interactions influence film characteristics and performance.
We have shown that in contrast to prevalent solvent based casting, engineered drug particles in nano and micro sizes may be incorporated through slurry casting for achieving high quality films that have excellent drug content uniformity and lead to very fast release even from poorly water soluble drugs. Other attractive features include the ability to formulate drugs, including those that are highly water insoluble, for many important therapeutic applications, such as quick-effect pain management, fast-acting remedy for nausea and vomiting, medications for Alzheimer’s disease which often leads to dysphagia (inability to swallow tablets), combination therapies required for treating cancer or AIDS, and most importantly, addressing unmet therapeutics needs in pediatric formulations, e.g., drug delivery in neonates requiring flexible dosages depending on the weight of the infant, and in pediatric cancers.
A major impact of this work is the recognition of C-SOPS leadership in this field, based on our publications, presentations and know-how generated. This project will generate required regulatory science for strip-film based products. In this project, we will determine the impact of raw and intermediate material properties as well as key process parameters for the individual unit operations in solvent and slurry based film formulation approaches on key quality attributes during continuous strip-film manufacturing. In addition, we will develop experimentally validated models for the film drying process and film erosion-dissolution, to assist identification of critical quality attributes, the overall sources of variability, and failure modes, as affected by the process parameters and material properties. Finally, we will test the hypothesis that film based products manufactured through well-designed film processes and formulations can have RtR capability based on integrated in-line sensing and process analytical technology (PAT).
Future Plans
Future plans include examining formulations and dosages of targeted products and drug delivery systems. These activities will be leveraged with the development of regulatory science funded by a project from FDA. Strip film format has several advantages including rapid disintegration and dissolution in the oral cavity and increased bio-availability, and is well suited for uniform dose manufacturing of high potency and/or poorly water-soluble drugs. Therefore, it can be and needs to be further developed for applications such as pediatric and geriatric medicine, extended release, abuse-proof formulations, buccal delivery, as well as neo-natal therapeutic applications. Our initial focus will be on pediatric or geriatric (e.g. Alzheimer) applications and developing suitable dosage forms for selected drug candidates. Later, we will examine products related to pain management or bipolar disorders. Subsequent years, we will collaborate with C-SOPS faculty to develop buccal delivery systems. Specific examples and details of this project will be developed in collaboration with C-SOPS pharma faculty with required expertise.